[Solved] A 10.0 mL of 0.20 M polyprotic acid (HnA) is titrated with a standard solution of 0.200 M NaOH
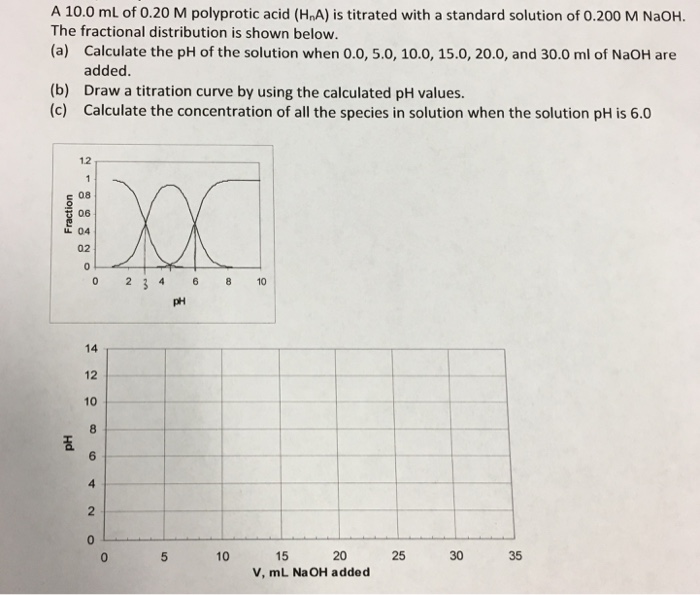
A 10.0 mL of 0.20 M polyprotic acid (HnA) is titrated with a standard solution of 0.200 M NaOH. The fractional distribution is shown below.
(a) Calculate the pH of the solution when 0.0, 5.0, 10.0, 15.0, 20.0, and 30.0 ml of NaOH are
(b) Draw a titration curve by using the calculated pH values. (c) Calculate the concentration of all the species in solution when the solution pH is 6.0 added. 1.2 c 08 06 04 02 0 2346 8 10 14 12 10 2 10 15 20 25 30 35 V, mL NaOH added
Expert Answer
Answer to A 10.0 mL of 0.20 M polyprotic acid (HnA) is titrated with a standard solution of 0.200 M NaOH….
OR